
While this does not constitute the final definition which ultimately will be decided through rules issued by CMS, endorsement by ONC is a major step forward. Expect the CMS draft rules this fall and final rules this winter.
The three adoption year progression of meaningful use includes:
- 2011 Data capture and sharing
- 2013 Advanced care processes with decision support
- 2015 Improved outcomes.
 Sure to confuse is the recommendation that the 2011 recommendations apply to the "adoption year" by the healthcare organization (HCO). In other words, if the HCO first adopts the EHR in 2012, that would be the year in which the 2011 definitions would apply. 2013 definitions would apply to the third adoption year.
Sure to confuse is the recommendation that the 2011 recommendations apply to the "adoption year" by the healthcare organization (HCO). In other words, if the HCO first adopts the EHR in 2012, that would be the year in which the 2011 definitions would apply. 2013 definitions would apply to the third adoption year.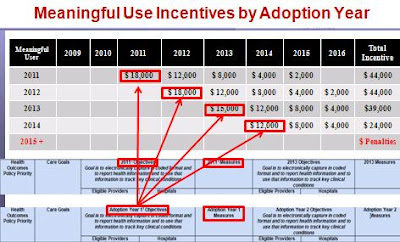 The workgroup responded to comments on their earlier proposals:
The workgroup responded to comments on their earlier proposals:- establishing a lower 10% threshhold for hospital CPOE adoption
- accelerating the intial use of clinical decision support (“Implement one clinical decision rule relevant to high clinical priority”)
- accelerating (to 2011) patients' access to their health information
- improving outcomes measures for care coordination by including a (2012 to 2013) measure of a 10% reduction on 30-day hospital readmissions
- withholding Medicare incentive payments until any HIPAA violations are resolved (and for state privacy and security violations, withholding incentives under Medicaid)
Of particular interest is the Meaningful Use Workgroup's concern over Health Information Exchange. They questioned how health information exchange was to be accomplished in 2011 when HIE organizations do not currently exist or do not connect all clinical trading partners. The workgroup deferred to the HIE workgroup for specific requirements and recommended that the 2015 targets include required participation in nationwide health information exchange.
The Meaningful Use Workgroup presented a 10 page Meaningful Use Matrix which summarizes the health outcomes policy priorities, care goals, objectives and measures by adoption year.
The Workgroup established 5 Health Outcomes Policy Priorities and documented the related Care Goals including:
1. Improve quality, safety, efficiency and reduce health disparities
- provide access to comprehensive patient health data for patient's health care team
- use evidence-based order sets and CPOE
- apply clinical decision support at the point of care
- generate lists of patients who need care and use them to reach out to patients (e.g., reminders, care instructions, etc.)
- report to patient registries for quality improvement, public reporting etc.
2. Engage patients and families
- provide patients and families with timely access to data, knowledge, and tools to make informed decisions and to manage their health
3. Improve care coordination
- exchange meaningful clinical information among professional health care team
4. Improve population and public health
- communicate with public health agencies
5. Ensure adequate privacy and security protections for personal health information
- ensure privacy and security protections for confidential information through operating policies, procedures, and technologies and compliance with applicable law
- provide transparency of dta sharing to patient
Objectives and Measures
For each of the Health Outcomes Policy Priorities, the Workgroup established related objectives and measures. The objectives were set separately for providers and hospitals
Providers' objectives for 2011 include:
Providers' objectives for 2011 include:
Improve quality, safety, efficiency and reduce health disparities
- use CPOE for all orders
- implement drug-drug, drug-allergy, drug-formulary checks
- maintain an up-to-date problem list of current and active diagnoses based on ICD-9 or SNOMED
- generate and transmit permissible prescriptions electronically (eRx)
- maintain active medication list
- maintain active medication allergy list
- record demographics including preferred language, insurance type, gender, race and ethnicity
- record advance directives
- record vital signs including height, weight, blood pressure
- calculate and display BMI
- record smoking status
- incorporate lab-test results into EHR as structure data
- generate lists of patients by specific conditions to use for quality improvement, reduction of disparities and outreach
- report ambulatory quality measures to CMS
- send reminders to patients per patient preference for preventive/follow up care
- implement one clinical decision rule relevant to specialty or high clinical priority
- document a progress note for each encounter
- check insurance eligibility electronically from public and private payers, where possible
- submit claims electronically to public and private payers
- provide patients with an electronic copy of their health information including lab results, problem list, medication lists, allergies) upon request
- provide patients with timely electronic access to their health information including lab results, problem list, medication lists, allergies
- provide access to patient-specific education resources
- provide clinical summaries for patients for each encounter
- capability to exchange key clinical information (e.g., problem list, medication list, allergies, test results), among providers of care and patient authorized entities electronically
- perform medication reconciliation at relevant encounters and each transition of care
- capability to submit electronic data to immunization registries and actual submission where required and accepted
- capability to provide electronic syndromic surveillance dtaa to public health agencies and actual transmission according to applicable law and practices
- compliance with HIPAA Privacy and Security rules
- compliance with fair data sharing practices set forth in the Nationwide Privacy and Security Framework
| 2011 Measures | NQF Endorsed Measures | |
| % diabetics with A1c under control [OP] | Title: Comprehensive Diabetes Care: HbA1c control (<8.0%)> | |
| % of hypertensive patients with BP under control [OP] | Title Controlling High Blood Pressure* | |
| % of patients with LDL under control [OP] | Title: IVD: Complete Lipid Profile and LDL Control <100 | |
| % of smokers offered smoking cessation counseling [OP, IP] | Title: Measure pair -a. Tobacco use prevention for infants, children and adolescents, b. Tobacco use cessation for infants, children and adolescents* | |
| % of patients with recorded BMI | Title: Body Mass Index (BMI) 2 through 18 years of age* | |
| [OP] | Title: Adult weight screening and follow up | |
| % eligible surgical patients who received VTE prophylaxis [IP] | Title: Surgery Patients Who Received Appropriate Venous Thromboembolism (VTE) Prophylaxis Within 24 Hours Prior to Surgery to 24 Hours After Surgery End Time* | |
| % of orders entered directly by physicians through CPOE | No current measures | |
| % of permissible RX's | Title Adoption of Medication e-Prescribing | |
| transmitted electronically | Title Medical Home System Survey | |
| % of med/all orders entered into | Title Adoption of Medication e-Prescribing | |
| CPOE | Title Medical Home System Survey | |
| Use of high-risk medications in the elderly [OP, IP] | Title: Drugs to be avoided in the elderly: a. Patients who receive at least one drug to be avoided, b. Patients who receive at least two different drugs to be avoided.* | |
| % of patients over 50 with annual colorectal cancer screenings [OP] | PQRI 113: Preventive Care and Screening: Colorectal Cancer Screening | |
| % of females over 50 receiving annual mammogram [OP] | PQRI 112: Preventive Care and Screening: Screening Mammography [PQRI age range 40-69] | |
| % patients at high-risk for cardiac events on aspirin prophylaxis [OP] | Title: Ischemic Vascular Disease (IVD): Use of Aspirin or another Antithrombotic.* | |
| % of patients with current pneumovax [OP] | PQRI 111: Preventive Care and Screening: Pneumonia Vaccination for Patients 65 Years and Older | |
| Title: Pneumococcal Vaccination of Nursing Home/ Skilled Nursing Facility Residents Description: Percent of nursing home/skilled nursing facility residents whose pneumococcal polysaccharide vaccine (PPV) status is up to date during the 12-month reporting period. | ||
| % eligible patients who received flu vaccine [OP] | PQRI -110: Preventive Care and Screening: Influenza Immunization for Patients . 50 Years | |
| Title: Influenza Vaccination of Nursing Home/ Skilled Nursing Facility Residents. | ||
| % lab results incorporated into EHR in coded format [OP,IP] | Title: The Ability for Providers with HIT to Receive Laboratory Data Electronically | |
| Title Medical Home System Survey | ||
| Stratify reports by gender, insurance type, primary language, race, ethnicity [OP, IP] | NQF has identified quality measurement criteria for which there are known disparities. CMS can use these criteria for stratification. | |
| % of all patients with access to personal health information electronically [OP, IP] | Title Medical Home System SurveyDescription | |
| % of all patients with access to patient specific educational resources [OP, IP] | No current measures | |
| % of encounters for which clinical summaries were provided [OP, IP] | No current measures | |
| Report 30-day readmission rate [IP] | Title: All-Cause Readmission Index (risk adjusted)*Title: All-Cause Readmission Index (risk adjusted)* | |
| % of encounters where med reconciliation was performed [OP, IP] | Title: Medication Reconciliation * | |
| Implemented ability to exchange health information with external clinical entity (specifically labs, care summary and medication lists) [OP, IP] | Title Medical Home System Survey | |
| Report up-to-date status for childhood immunizations [OP] | Title: Childhood Immunization Status * | |
| % reportable lab results submitted electronically [IP] | No current measures | |
| Provide electronic syndrome surveillance data to public health agencies according to applicable law and practice [IP] | No current measures | |
| Full compliance with HIPAA Privacy and Security Rules | No current measures | |
| An entity under investigation for a HIPAA privacy or security violation cannot achieve meaningful use until the entity is cleared by the investigating authority | No current measures | |
| Conduct or update a security risk assessment and implement security updates as necessary | No current measures | |
| Other Measures Under Consideration (In addition to initial Policy Measure 2001 Grid) | PQRI 7: Coronary Artery Disease (CAD): Beta-Blocker Therapy for CAD Patients with Prior Myocardial Infarction (MI)* | |
| Other Measures Under Consideration(In addition to initial Policy Measure 2001 Grid) | PQRI 5: Heart Failure: Angiotensin-Converting Enzyme (ACE) Inhibitor or Angiotensin Receptor Blocker (ARB) Therapy for Left Ventricular Systolic Dysfunction (LVSD)* | |
| Other Measures Under Consideration (In addition to initial Policy Measure 2001 Grid) | Title: Use of appropriate medications for people with asthma | |
| Other Measures Under Consideration(In addition to initial Policy Measure 2001 Grid) | Title: Patients with Atrial Fibrillation Receiving Anticoagulation Therapy Description |








1 comment:
One of the best explanations I have read so far! Especially clear on the "adoption year"
Post a Comment