The DEA published the following FAQ's for
electronic prescriptions for controlled substances by practitioners. Highlights follow:
Questions and Answers for Prescribing Practitioners
Q. What is DEA’s rule “Electronic Prescriptions for Controlled Substances?”
A. DEA’s rule, “Electronic Prescriptions for Controlled Substances” revises DEA’s regulations to provide practitioners with the option of writing prescriptions for controlled substances electronically. The regulations will also permit pharmacies to receive, dispense, and archive these electronic prescriptions. The rule was published in the Federal Register Wednesday, March 31, 2010 and becomes effective on June 1, 2010.
Q. Is the use of electronic prescriptions for controlled substances mandatory?
A. No, the new regulations do not mandate that practitioners prescribe controlled substances using only electronic prescriptions. Nor do they require pharmacies to accept electronic prescriptions for controlled substances for dispensing. Whether a practitioner or pharmacy uses electronic prescriptions for controlled substances is voluntary from DEA’s perspective. Prescribing practitioners are still able to write, and manually sign, prescriptions for schedule II, III, IV, and V controlled substances and pharmacies are still able to dispense controlled substances based on those written prescriptions. Oral prescriptions remain valid for schedule III, IV, and V controlled substances. Electronic prescriptions for controlled substances are only permissible if the electronic prescription and the pharmacy application meet DEA’s requirements. In addition, electronic prescriptions for controlled substances may be subject to state laws and regulations. If state requirements are more stringent than DEA’s regulations, the state requirements would supersede any less stringent DEA provision.
Q. When can a practitioner start issuing electronic prescriptions for controlled substances?
A. A practitioner will be able to issue electronic controlled substance prescriptions only when the electronic prescription or electronic health record (EHR) application the practitioner is using complies with the requirements in the interim final rule.
Q. How will a practitioner be able to determine that an application complies with DEA’s rule?
A. The application provider must either hire a qualified third party to audit the application or have the application reviewed and certified by an approved certification body. The auditor or certification body will issue a report that states whether the application complies with DEA’s requirements and whether there are any limitations on its use for controlled substance prescriptions. (A limited set of prescriptions require information that may need revision of the basic prescription standard before they can be reliably accommodated, such as hospital prescriptions issued to staff members with an identifying suffix.) The application provider must provide a copy of the report to practitioners who use or are considering use of the electronic prescription application to allow them to determine whether the application is compliant with DEA’s requirements.
Q. Until a practitioner has received an audit/certification report from the application provider indicating that the application meets DEA's requirements, how can the electronic prescription application or electronic health record application be used to write controlled substances prescriptions?
A. Nothing in this rule prevents a practitioner or a practitioner's agent from using an existing electronic prescription or EHR application that does not comply with the interim final rule to prepare and print a controlled substance prescription, so that EHR and other electronic prescribing functionality may be used. Until the application is compliant with the final rule, however, the practitioner will have to print the prescription for manual signature. Such prescriptions are paper prescriptions and subject to the existing requirements for paper prescriptions.
Individual Practitioners: Getting Started
(Note: The questions and responses below assume that the practitioner is an individual practitioner (e.g., physician, dentist, veterinarian, nurse practitioner) and is a DEA registrant lawfully permitted to prescribe controlled substances. The practitioner may be a member of a group practice. They further assume that the practitioner has received an audit or certification report from the application provider of the practitioner’s software used to create prescriptions for controlled substances that indicates the application meets DEA’s requirements.)
Q. Is identity proofing of individual prescribing practitioners required. If so, who will conduct it?
A. Yes, identity proofing is critical to the security of electronic prescribing of controlled substances. Authentication credentials used to sign controlled substance prescriptions may be issued only to individuals whose identity has been confirmed. Individual practitioners will be required to apply to certain Federally approved credential service providers (CSPs) or certification authorities (CAs) to obtain their two-factor authentication credential or digital certificate. The CSP or CA will be required to conduct identity proofing that meets National Institute of Standards and Technology Special Publication 800-63-1 Assurance Level 3. Both in person and remote identity proofing will be acceptable.
Q. If a practitioner wants to undergo identity proofing to prescribe controlled substances, how is this accomplished?
A. DEA expects application providers will work with CSPs or CAs to direct practitioners to one or more sources of two-factor authentication credentials that will be interoperable with their applications. Prescribing practitioners may wish to contact their application provider to determine which CSP or CA the provider recommends the practitioner use. The specifics of each application will determine what kind of two-factor credential will be needed.
Q. Is remote identity proofing permissible?
A. Yes, the rule permits both in-person and remote identity proofing. DEA believes that the ability to conduct remote identity proofing allowed for in National Institute of Standards and Technology Special Publication 800-63-1 Level 3 will ensure that practitioners in rural areas will be able to obtain an authentication credential without the need for travel.
Q. Once a practitioner has undergone identity proofing, will the practitioner receive something?
A. The CSP or CA that conducted the identity proofing of the practitioner may issue a new hard token or register and provide credentials for an existing token. Regardless of whether a new token is provided and activated, an existing token is registered, or a biometric is used for the signing of controlled substance prescriptions, communications between the CSP or CA and practitioner applicant must occur through two channels (e.g., mail, telephone, e-mail).
Q. Why is DEA requiring the use of two-factor authentication credentials?
A. Two-factor authentication (two of the following – something you know, something you have, something you are) protects the practitioner from misuse of his/her credential by insiders as well as protecting him/her from external threats because the practitioner can retain control of a biometric or hard token. Authentication based only on knowledge factors is easily subverted because they can be observed, guessed, or hacked and used without the practitioner’s knowledge.
Q. What two-factor credentials will be acceptable?
A. Under the interim final rule, DEA is allowing the use of two of the following – something you know (a knowledge factor), something you have (a hard token stored separately from the computer being accessed), and something you are (biometric information). The hard token, if used, must be a cryptographic device or a one-time password device that meets Federal Information Processing Standard 140-2 Security Level 1.
Q. What is a hard token?
A. A hard token is a cryptographic key stored on a hardware device (e.g., a PDA, cell phone, smart card, USB drive, one-time password device) rather than on a general purpose computer. A hard token is a tangible, physical object possessed by an individual practitioner.
Q. Is it permissible for an individual practitioner to have the office manager or other staff maintain custody of the individual practitioner’s hard token?
A. No, the practitioner must retain sole possession of the hard token, where applicable, and must not share the password or other knowledge factor with any other person. The practitioner must not allow any other person to use the token or enter the knowledge factor or other identification means to sign prescriptions for controlled substances. Failure by the practitioner to secure the hard token or knowledge factor may provide a basis for revocation or suspension of the practitioner’s DEA registration.
Q. If an individual practitioner wants to use a biometric as one factor of the two-factor authentication credential, does DEA have any special requirements?
A. DEA is establishing several standards for the use of biometrics and for the testing of the software used to read the biometrics. DEA wishes to emphasize that these standards do not specify the types of biometrics that may be acceptable. Any biometric that meets the criteria DEA has specified may be used as the biometric factor in a two-factor authentication credential used to indicate that prescriptions are ready to be signed and sign controlled substance prescriptions. The use of biometrics as one factor in the two-factor authentication protocol is strictly voluntary, as is all electronic prescribing of controlled substances.
Q. Does an individual practitioner need separate authentication credentials if the practitioner has more than one DEA registration?
A. No, a single authentication credential can be used. The practitioner or the practitioner’s agent must, however, select the appropriate DEA registration number when the prescription is created.
Q. If an individual practitioner uses more than one application to create and sign controlled substance prescriptions, will the practitioner need to undergo identity proofing for each and obtain separate credentials for each?
A. Whether the individual practitioner needs to undergo identity proofing and obtain separate credentials for separate applications will depend on the requirements of the applications. It is likely that if a practitioner has privileges at one or more hospitals, the hospitals will require separate credentials to use their applications.
Q. Once a practitioner possesses the two-factor credential, is the practitioner ready to sign controlled substance prescriptions?
A. No, there is another step that must be taken. Any application that meets DEA’s requirements will require the practice to set access controls so that only individuals legally authorized to sign controlled substance prescriptions are allowed to do so. The application will determine whether access control is set by name or by role. If the logical access controls are role-based, one or more roles will have to be limited to individuals authorized to prescribe controlled substances. This role may be labeled “DEA registrant” or physician, dentist, nurse practitioner, etc.
Q. How are access controls set?
A. Setting access controls requires two people. One person must determine which individuals are authorized to sign controlled substance prescriptions and enter those names or assign those names to a role that is allowed to sign controlled substance prescriptions. A DEA registrant must then use his/her two-factor credential to execute the access control list. The access control list will need to be updated when registrants join or leave a practice.
Q. Who has to determine whether a prescribing practitioner’s DEA registration is current and in good standing?
A. A person at the practice who is setting access control has to check to be sure that each practitioner being granted authorization to sign controlled substances prescriptions has a DEA registration, state authorization to practice and, where applicable, state authorization to dispense controlled substances that are still current and in good standing. DEA expects this will be done simply by checking the latest certificates.
Institutional Practitioners: Getting Started
(Note: The questions and responses below assume that the practitioner is an institutional practitioner (e.g., a hospital or clinic) and is a DEA registrant lawfully permitted to prescribe controlled substances. They further assume that the practitioner has received an audit or certification report from the application provider of the practitioner’s software used to create prescriptions for controlled substances that indicates the application meets DEA’s requirements.)
Q. Is identity proofing required for any individual practitioner whom the institutional practitioner is granting access to issue prescriptions using the institution’s electronic prescribing application? If so, who will conduct it?
A. Yes, as identity proofing is critical to the security of electronic prescribing of controlled substances. Authentication credentials used to sign controlled substance prescriptions are issued only to individuals whose identity has been confirmed. DEA is allowing institutional practitioners, who are DEA registrants, to conduct the identity proofing for any individual practitioner whom the institutional practitioner is granting access to issue prescriptions using the institution’s electronic prescribing application. Because institutional practitioners have credentialing offices, those offices may conduct in-person identity proofing as part of the credentialing process. DEA is not requiring institutional practitioners to meet the requirements of National Institute of Standards and Technology Special Publication 800-63-1 for identity proofing. Before the institutional practitioner issues the authentication credential, a person designated by the institutional practitioner must check the individual practitioner’s government-issued photographic identification against the person presenting it. The institutional practitioner must also check State licensure and DEA registrations, where applicable.
Q. Is an institutional practitioner required to conduct identity proofing in this manner?
A. No, institutional practitioners are allowed, but not required, to conduct identity proofing. If an institutional practitioner decides to have each practitioner obtain identity proofing and the two-factor authentication credential on his own, as other individual practitioners do, that is permissible under the rule.
Q. For an institutional practitioner, is remote identity proofing permissible?
A. The rule only allows institutional practitioners to conduct in-person identity proofing. Remote identity proofing is not permissible for institutional practitioners.
Q. For an institutional practitioner, how is the two-factor authentication credential issued?
A. Under the rule, the institutional practitioner may issue the two-factor authentication credentials or obtain them from a third party which will have to be a CSP or CA that meets the criteria DEA has specified. In the latter case, the institutional practitioner could have each practitioner apply for the two-factor credential himself, which would entail undergoing identity proofing by the CSP or CA. Alternatively, the institutional practitioner can serve as a trusted agent for the third party. Trusted agents conduct part of the identity proofing on behalf of the CSP or CA and submit the information for each person along with a signed agreement that specifies the trusted agent’s responsibilities.
Q. Why is DEA requiring the use of two-factor authentication credentials?
A. Two-factor authentication (two of the following – something you know, something you have, something you are) protects the practitioner from misuse of his/her credential by insiders as well as protecting him/her from external threats because the practitioner can retain control of a biometric or hard token. Authentication based only on knowledge factors is easily subverted because they can be observed, guessed, or hacked and used without the practitioner’s knowledge.
Q. What two-factor credentials will be acceptable?
A. Under the interim final rule, DEA is allowing the use of two of the following – something you know (a knowledge factor), something you have (a hard token stored separately from the computer being accessed), and something you are (biometric information). The hard token, if used, must be a cryptographic device or a one-time-password device that meets Federal Information Processing Standard 140-2 Security Level 1.
Q. What is a hard token?
A. A hard token is a cryptographic key stored on a hardware device (e.g., a PDA, cell phone, smart card, USB drive, one-time password device) rather than on a general purpose computer. A hard token is a tangible, physical object possessed by an individual practitioner.
Q. Is it permissible for a practitioner to have another staff person at the institutional practitioner maintain custody of the hard token?
A. No, the practitioner must retain sole possession of the hard token, where applicable, and must not share the password or other knowledge factor with any other person. The practitioner must not allow any other person to use the token or enter the knowledge factor or other identification means to sign prescriptions for controlled substances.
Q. If an institutional practitioner wants to use a biometric as one factor of the two-factor authentication credential issued to persons prescribing controlled substances, does DEA have any special requirements?
A. DEA is establishing several standards for the use of biometrics and for the testing of the software used to read the biometrics. DEA wishes to emphasize that these standards do not specify the types of biometrics that may be acceptable. Any biometric that meets the criteria DEA has specified may be used as the biometric factor in a two-factor authentication credential used to indicate that prescriptions are ready to be signed and sign controlled substance prescriptions. The use of biometrics as one factor in the two-factor authentication protocol is strictly voluntary, as is all electronic prescribing of controlled substances.
Q. Are any additional steps needed to give practitioners the ability to sign controlled substance prescriptions?
A. Yes, once a person’s identity has been confirmed by the credentialing office and a two-factor credential has been issued, another office must set access controls. The application must have the ability to assign permissions by name or role so that only authorized practitioners are allowed to sign controlled substance prescriptions. Two individuals must be involved in setting the access controls; one will enter the data based on information from the credentialing office and the second will approve the entry.
Accessing the Electronic Prescription Application or Electronic Health Record Application to Sign Controlled Substance Prescriptions
Q. When must a practitioner’s permission to indicate that controlled substance prescriptions are ready to be signed and sign controlled substance prescriptions be revoked?
A. A practitioner’s permission to indicate that controlled substance prescriptions are ready to be signed and to sign controlled substance prescriptions must be revoked whenever any of the following occurs, on the date it is discovered:
- If a hard token or any other authentication factor required by the two-factor authentication protocol is lost, stolen, or compromised. Such access must be terminated immediately upon receiving notification from the individual practitioner.
- The individual practitioner’s DEA registration expires, unless the registration has been renewed.
- For individual practitioners prescribing controlled substances under the registration of an institutional practitioner, when the institutional practitioner’s DEA registration expires, unless the registration has been renewed.
- The individual practitioner’s DEA registration is terminated, revoked, or surrendered.
- For individual practitioners prescribing controlled substances under the registration of an institutional practitioner, when the institutional practitioner’s DEA registration is terminated, revoked, or surrendered.
- The individual practitioner is no longer authorized to use the electronic prescription application (e.g., when the individual practitioner leaves the practice).
- When an individual practitioner is no longer authorized to use the institutional practitioner’s electronic prescription application (e.g., when the individual practitioner is no longer associated with the institutional practitioner).
Creating and Signing Prescriptions
Q. What information is an electronic prescription for a controlled substance required to contain?
A. As with paper prescriptions, all electronic prescriptions for controlled substances are required to contain the full name and address of the patient, drug name, strength, dosage form, quantity prescribed, directions for use, and the name, address and registration number of the practitioner. The prescription shall be dated as of the day when signed and shall be signed by the practitioner using his/her two-factor authentication credential. Where applicable, refill information must also be included, as well as any other information required by DEA regulations.
Q. Is a practitioner required to review a prescription before signing it?
A. All controlled substances must be reviewed by the prescribing practitioner. The practitioner must affirmatively indicate those prescriptions that are ready to be signed. A practitioner has the same responsibility when issuing an electronic prescription as when issuing a paper prescription to ensure that the prescription conforms in all respects with the requirements of the Controlled Substances Act and DEA regulations. This responsibility applies with equal force regardless of whether the prescription information is entered by the practitioner or a member of his staff.
Q. When a practitioner reviews a prescription, what information must be displayed?
A. All information required of any controlled substance prescription must be displayed, except for the patient’s address. However, the patient’s address must be part of the elements of the prescription that are digitally signed by the practitioner or the application and transmitted to the pharmacy.
Q. Must a practitioner separately attest to each prescription?
A. No, the application must include, on the prescription review screen, the following statement or its substantial equivalent: “By completing the two-factor authentication protocol at this time, you are legally signing the prescription(s) and authorizing the transmission of the above information to the pharmacy for dispensing. The two-factor authentication protocol may only be completed by the practitioner whose name and DEA registration number appear above.” However, no keystroke is required to acknowledge the statement.
Q. Is it permissible to have a staff person in the practitioner’s office complete all of the required information for a controlled substance prescription and then have the practitioner review, sign, and authorize the transmission of the prescription?
A. Yes, however, if an agent of the practitioner enters information at the practitioner’s direction prior to the practitioner reviewing and approving the information, the practitioner is responsible in the event the prescription does not conform in all essential respects to the law and regulations.
Q. How will the two-factor credential be used?
A. The practitioner will use the two-factor credential to sign the prescription; that is, using the two-factor credential will constitute the legal signature of the DEA-registered prescribing practitioner. When the credential is used, the application must digitally sign and archive at least the DEA-required information contained in the prescription.
Q. May a practitioner use his/her own digital certificate to sign an electronic controlled substance prescription?
A. Yes, the interim final rule allows any practitioner to use his/her own digital certificate to sign electronic prescriptions for controlled substances. If the practitioner and his/her application provider wish to do so, the two-factor authentication credential can be a digital certificate specific to the practitioner that the practitioner obtains from a certification authority that is cross-certified with the Federal Bridge Certification Authority at the basic assurance level.
Q. How is an electronic controlled substance prescription signed?
A. The prescribing practitioner whose name and DEA registration number appear on the prescription must indicate those controlled substance prescriptions that are ready to be signed. When the registrant indicates that one or more prescriptions are to be signed, the application must prompt him/her to begin a two-factor authentication protocol. Completion of the two-factor authentication protocol legally signs the prescription(s).
Q. Will a practitioner be allowed to simultaneously issue multiple prescriptions for multiple patients with a single signature?
A. A practitioner is not permitted to issue prescriptions for multiple patients with a single signature.
Q. If a practitioner is signing more than one controlled substance prescription for a single patient, how many executions of the two-factor authentication protocol are required?
A. Each controlled substance prescription will have to be indicated as ready for signing, but execution of a single two-factor authentication protocol can then sign all prescriptions for a given patient.
Q. Once an electronic controlled substance prescription is signed, must it be transmitted to the pharmacy immediately?
A. No, signing and transmitting an electronic controlled substance prescription are two distinct actions. Electronic prescriptions for controlled substances should be transmitted as soon as possible after signing, however, it is understood that practitioners may prefer to sign prescriptions before office staff add pharmacy or insurance information. Therefore, DEA is not requiring that transmission of the prescription occur simultaneously with signing the prescription.
Other Issues
Q. If a mid-level practitioner practices in a state that requires the controlled substance prescription to contain the mid-level practitioner’s supervisor’s DEA number as well as the mid-level practitioner’s DEA number, is this possible with electronic controlled substance prescriptions?
A. Multiple DEA numbers can appear on a single prescription, if required by state law or regulations, provided that the electronic prescription application clearly identifies which practitioner is the prescriber and which is the supervisor.
Q. Practitioners who work in a group practice with multiple practitioners may have all of the practitioners’ names printed on the practice’s prescription pads. Can all of the practitioners’ names appear on the practice’s electronic controlled substance prescriptions?
A. No, for electronic prescriptions, only one prescribing practitioner’s name and DEA number will appear. If a practitioner needs to sign a prescription originally created and indicated as ready for signing by another practitioner in a practice, he/she must change the practitioner name and DEA number to his/her own. The only exception to this rule is if required by state law or regulations, multiple DEA numbers can appear on a single prescription provided that the electronic prescription application clearly identifies which practitioner is the prescriber and which is the supervisor.
Q. Can a qualified practitioner who prescribes schedules III, IV, and V narcotic controlled drugs approved by the Food and Drug Administration specifically for use in maintenance or detoxification treatment use electronic prescriptions for controlled substances for this purpose?
A. Yes, a qualified practitioner may use electronic prescriptions for controlled substances to prescribe schedules III, IV, and V narcotic controlled drugs approved by the Food and Drug Administration specifically for use in maintenance or detoxification treatment if the audit or certification report the practitioner receives from the application provider specifically states that the application meets DEA’s requirements for those prescriptions.
Q. How can a practitioner obtain his/her prescribing history?
A. DEA is requiring that the electronic prescription application be able to generate a log, upon request by the practitioner, of all electronic prescriptions for controlled substances the practitioner issued using the application over at least the preceding two years. This log is required to be sortable at least by patient name, drug name, and date of issuance.
Transmitting Prescriptions to the Pharmacy and Printing Prescriptions
Q. What is an intermediary?
A. An intermediary means any technology system that receives and transmits an electronic prescription between the practitioner and the pharmacy.
Q. If transmission of an electronic prescription fails, may the intermediary convert the electronic prescription to another form (e.g. facsimile) for transmission?
A. No, an electronic prescription must be transmitted from the practitioner to the pharmacy in its electronic form. If an intermediary cannot complete a transmission of a controlled substance prescription, the intermediary must notify the practitioner. Under such circumstances, if the prescription is for a schedule III, IV, or V controlled substance, the practitioner can print the prescription, manually sign it, and fax the prescription directly to the pharmacy. This prescription must indicate that it was originally transmitted to, and provide the name of, a specific pharmacy, the date and time of transmission, and the fact that the electronic transmission failed.
Q. What are the DEA requirements regarding the storage of electronic prescription records?
A. Once a prescription is created electronically, all records of the prescription must be retained electronically. As is the case with paper prescription records, electronic controlled substance prescription records must be kept for a minimum period of two years.
Reporting Security Incidents
Q. Is a person who administers logical access controls required to report security incidents?
A. Yes, the application is required to run an internal audit for potential security incidents daily and generate a report of any such incidents. If the application generates a report and, upon investigation, the person(s) designated to administer logical access controls for the practice or institutional practitioner determines that the issuance or records of controlled substance prescriptions has been compromised or could have been compromised, it must be reported to the application provider and DEA within one business day. In general, the security incidents that should be reported are those that represent successful attacks on the application or other incidents in which someone gains unauthorized access.
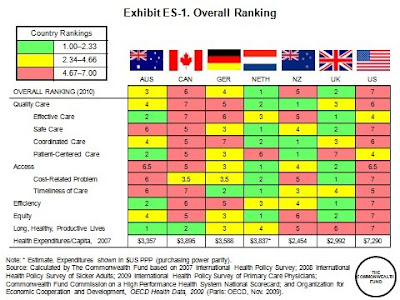 But even when access and equity measures are not considered, the U.S. ranks behind most of the other countries on most measures. With the inclusion of primary care physician survey data in the analysis, it is apparent that the U.S. is lagging in adoption of national policies that promote primary care, quality improvement, and information technology. Health reform legislation addresses these deficiencies; for instance, the American Recovery and Reinvestment Act signed by President Obama in February 2009 included approximately $19 billion to expand the use of health information technology. The Patient Protection and Affordable Care Act of 2010 also will work toward realigning providers’ financial incentives, encouraging more efficient organization and delivery of health care, and investing in preventive and population health.
But even when access and equity measures are not considered, the U.S. ranks behind most of the other countries on most measures. With the inclusion of primary care physician survey data in the analysis, it is apparent that the U.S. is lagging in adoption of national policies that promote primary care, quality improvement, and information technology. Health reform legislation addresses these deficiencies; for instance, the American Recovery and Reinvestment Act signed by President Obama in February 2009 included approximately $19 billion to expand the use of health information technology. The Patient Protection and Affordable Care Act of 2010 also will work toward realigning providers’ financial incentives, encouraging more efficient organization and delivery of health care, and investing in preventive and population health.












